What are nuclear weapons? How are they made? What scientific concepts underlie them? Below is a glossary of key terms to know when reading articles on this website, or any article about nuclear technology and weapons.

The Science Behind Fission and Fusion
Fission is a type of nuclear reaction that involves splitting an atom’s nucleus into smaller nuclei. It can be induced or may occur naturally through radioactive decay.
Fusion is a type of nuclear reaction that involves combining of two smaller nuclei into a larger nucleus. This process occurs naturally in stars and may be induced at very high temperatures.
The principle behind fission and fusion lies in the composition of the nucleus itself. The nucleus of any atom can contain two kinds of subatomic particles, neutrons and protons (collectively called nucleons), that are held together by the strong force.
The strong force is one of the four fundamental forces of nature. In addition to holding nucleons together in a nucleus, it is also responsible for the mere existence of nucleons. Unlike what high school science may introduce, protons and neutrons are not the smallest building blocks of matter – they are composed of even smaller fundamental particles called quarks. The strong force binds quarks together to form nucleons, and also keeps neutrons and protons together in the nucleus.
Protons are positively charged, while neutrons are neutral in charge. As a result, protons repel each other and the strong force must be greater than this repulsive electrostatic force. As the number of protons in a nucleus increases, the number of neutrons and the amount of energy that keeps the nucleus together changes as well.
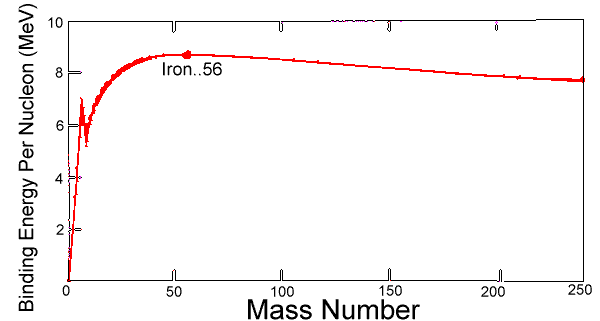
Nuclear Binding Energy is the amount of energy needed to disassemble a nucleus into individual protons and neutrons. This energy is reported per nucleon. Iron is the most tightly bound atom (requiring the most energy per nucleon to be disassembled). This means that anything lighter than iron will release energy upon fusion to become a bigger nucleus, and heaver nuclei will release energy upon fission to become smaller. In each case, resulting elements will approach iron in mass number. This is shown in Figure 1 below.
Fission Weapons
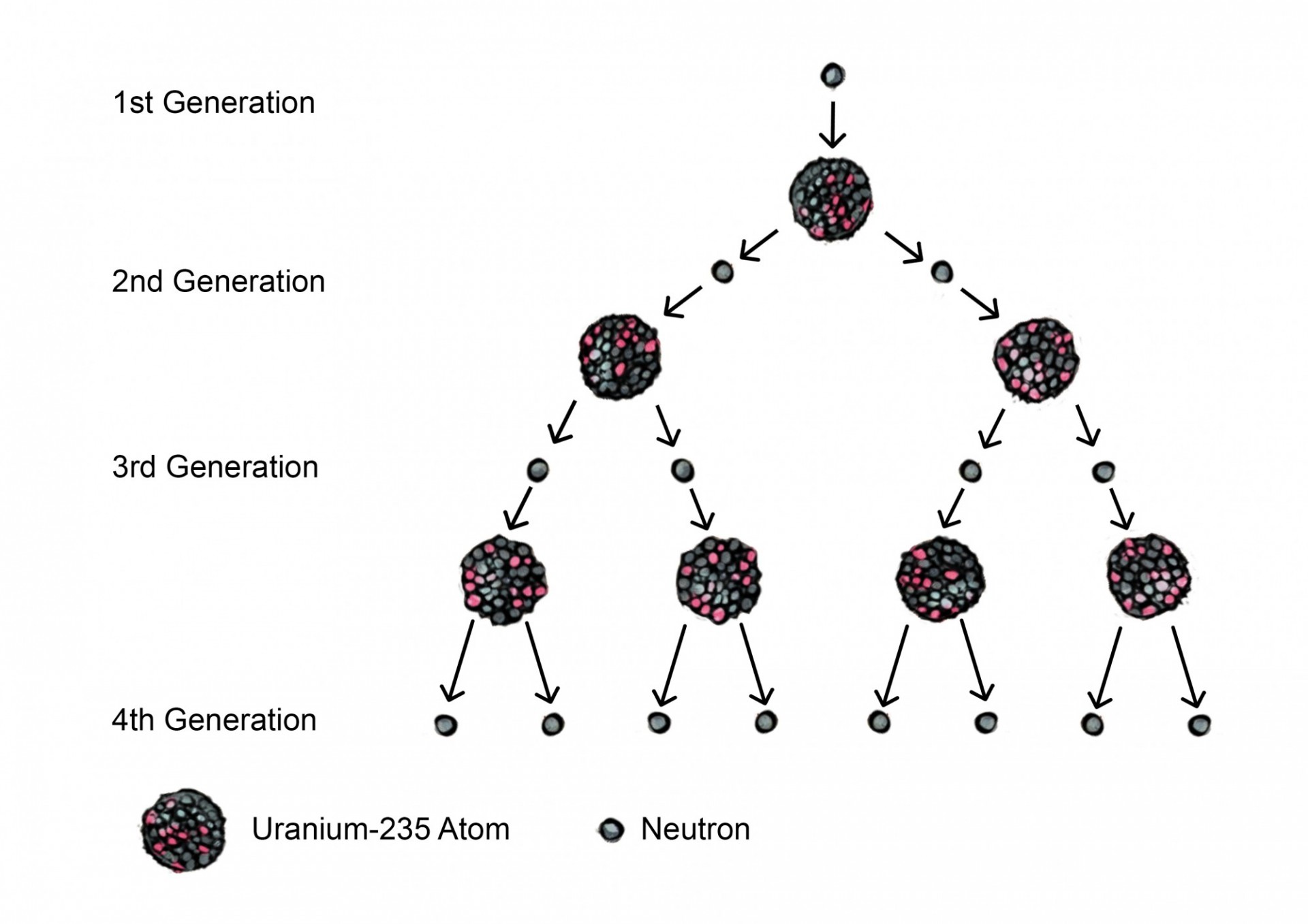
Uranium is a commonly used element for fission reactions. The most abundant isotope of uranium, uranium-238, is not fissile material because it cannot sustain a chain reaction. Uranium-235, however, is fissile – it can sustain a chain reaction using the neutrons that are produced from the fission of other Uranium-235 nuclei. For example, if a nuclear reaction in which one neutron bombards a Uranium-235 nucleus produces two neutrons, the resulting neutrons will be able to bombard other Uranium-235 nuclei in this material. See Figure 2.
Critical Mass is the mass of fissile material needed for a chain reaction to become self sustaining. The reason why such a minimum mass exists is that not every neutron that is produced from a fission reaction is going to go on and cause another reaction – some may escape before colliding with another uranium atom, or collide at insufficient speeds or the incorrect angle. Thus, a larger mass of material is needed to ensure that the chain reaction can continue. On the other hand, fusion materials don’t require a critical mass because they involve the merging of nuclei rather than their splitting, which is initiated by heat rather than collision with a neutron.
Highly Enriched Uranium, Uranium-235, only comprises 0.7% of an average sample of natural uranium, with the majority of natural uranium being uranium-238. In order for there to be enough Uranium-235 for controlled reactions to produce nuclear energy, the proportion of Uranium-235 has to be increased to 2-3% (low enriched uranium). To produce a nuclear bomb, the sample of uranium should contain about 90% Uranium-235. Samples of uranium with more than 20% concentration of Uranium-235 is what is meant by highly enriched uranium. The Little Boy bomb, dropped over Hiroshima, used about 64 kg of 80% enriched uranium. The more enriched the uranium is, the smaller the critical mass needed to create a weapon.
If Uranium-235 does not naturally occur in such high proportions, how do we increase the ratio of Uranium-235 to Uranium-238? Because they are chemically identical, the two isotopes must be separated by mass. One way of doing so is using centrifuges, which use the miniscule weight difference between the two isotopes to separate them. In a common gaseous centrifuge, the uranium first undergoes a chemical reaction that turns it into a gaseous state. Then, the uranium gas is spun very quickly in the centrifuge, so that the heavier Uranium-238 moves towards the edge and the lighter Uranium-235 moves towards the center. By repeating this process thousands of times using thousands of centrifuges, the uranium is finally sufficiently enriched to be used in the production of a nuclear weapon.

Plutonium-239 is a fissile isotope that is formed from the decay reaction of Uranium-238. It is also able to sustain a chain reaction, and thus can be used in the production of nuclear weapons.
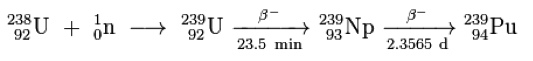
A Magnox Reactor is a very primitive type of reactor that uses natural uranium. Its name, magnox, is derived from the magnesium oxide alloy that is used to coat fuel rods. The uranium undergoes fission reaction in a controlled setting, and a large portion of it is transformed into plutonium.
Fusion Weapons
Hydrogen Bombs: As opposed to atomic bombs that rely on fission (splitting uranium and/or plutonium to release large amounts of energy), hydrogen bombs use fusion reactions to produce far more energy (per unit mass). In particular, a hydrogen bomb uses an initial nuclear fission explosion to create the conditions that allow for compression and fusion of deuterium and tritium (isotopes of hydrogen), near the heart of the bomb. The large amounts of neutrons set free by this process can ramp up the explosive chain reaction of a uranium layer wrapped around it, creating a blast far more powerful than uranium fission alone. Hydrogen bombs require a fission component to produce the high temperatures necessary for the nuclei to come together.
Boosted Fission Weapon: Unlike hydrogen bombs, where the fission reaction is used to initiate the fusion reaction from which most of the energy is derived, a boosted fission weapon uses a fusion reaction to increase the rate of the fission reaction. Within such a weapon, a smaller fusion reaction is used to produce more neutrons that would then go on to cause more fission chain reactions, substantially increasing the rate of the reaction. In such a process, the fusion reaction itself only contributes about an additional 1% of energy to the weapon. Rather, it is the increased rate and yield of the fission reaction that makes a boosted fission weapon more powerful, and allows for a smaller nuclear warhead.
Further reading:
Brain, Marshall. “What’s a uranium centrifuge?”, How Stuff Works, Oct 26, 2006.
Pappas, Stephanie. “Hydrogen Bomb v Atomic Bomb: What’s the Difference?”, Live Science, Sept 22, 2017.
“Nuclear Fission”, Nuclear Power, n.p.
“Uranium-235”, Nuclear Power, n.p.
Nave, R. “Nuclear Fusion”, Hyperphysics, n.p.
Sutton, Christine. “Strong Force”, Encyclopedia Brtannica, Feb 2, 2017.
Zielinski, Sarah. “What is Enriched Uranium?”, The Smithsonian, Jan 10, 2012.